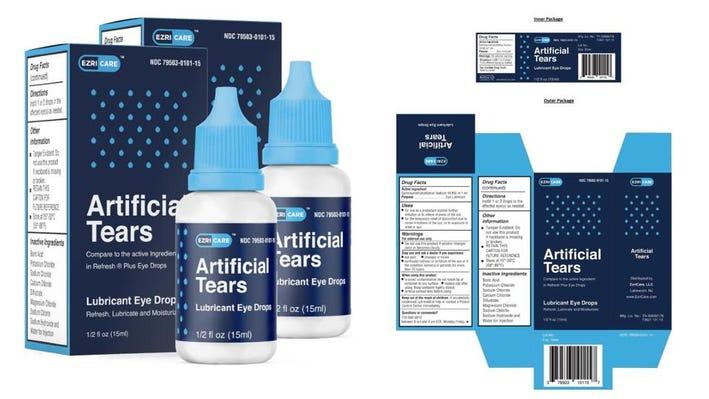
Global Pharma recalls Artificial Tears eye drops from US
Chennai based Global Pharma Healthcare has recalled ‘Artificial Tears’ eye drops from all its retailers and wholesalers in the US following an alert issued by the US Food and Drug Administration about 55 cases of adverse events.
The ‘Artificial Tears’ eye drop manufactured by Global Pharma Healthcare was under inquiry in 12 states of the US since May 2022.
“So far, there are 55 reports of adverse events including eye infections, permanent loss of vision, and a death with a bloodstream infection,” Global Pharma Healthcare said in its recall order, citing the Centers for Disease Control and Prevention.
Officials of the drug control department of Tamil Nadu said an investigation into the manufacturing plant has been launched on Friday.
The Centers for Disease Control and Prevention had collected samples from patients between May 2022 and January 2023, who used the eye drop and suffered from inflammation in the cornea, infection in the eyeball, respiratory infection, urinary tract infection, sepsis and vision loss.
On February 1, the Centers for Disease Control and Prevention issued an alert stating that the eye drops had been packaged under the name “EzriCare” and so far 12 states have reported 55 patients who purchased the eye drop over the counter.
This is the third Indian pharmaceutical company linked with mass casualties or adverse events in other countries since October 2022.
In October, the World Health Organisation issued a medical alert against four cough syrups manufactured by Haryana based Maiden Pharmaceuticals after linking it with death of 70 children in Gambia. In January, Uzbekistan government issued an alert against Uttar Pradesh based company, Marion Biotech, after finding contamination in anti cold syrups that led to death of 18 people.
